 Ordinary oil droplets in water are too big to move by Brownian motion driven by thermal fluctuations, however they can swim if they dissolve in their surroundings in a way that causes an asymmetry in the surfactants at the interface. Understanding this phenomenon is important because it allows us to tune key parameters, such as the droplet speed and turning frequency, but also because the locomotion of droplets mimics the chemotaxis of microorganisms in their own secretion.
Ordinary oil droplets in water are too big to move by Brownian motion driven by thermal fluctuations, however they can swim if they dissolve in their surroundings in a way that causes an asymmetry in the surfactants at the interface. Understanding this phenomenon is important because it allows us to tune key parameters, such as the droplet speed and turning frequency, but also because the locomotion of droplets mimics the chemotaxis of microorganisms in their own secretion.
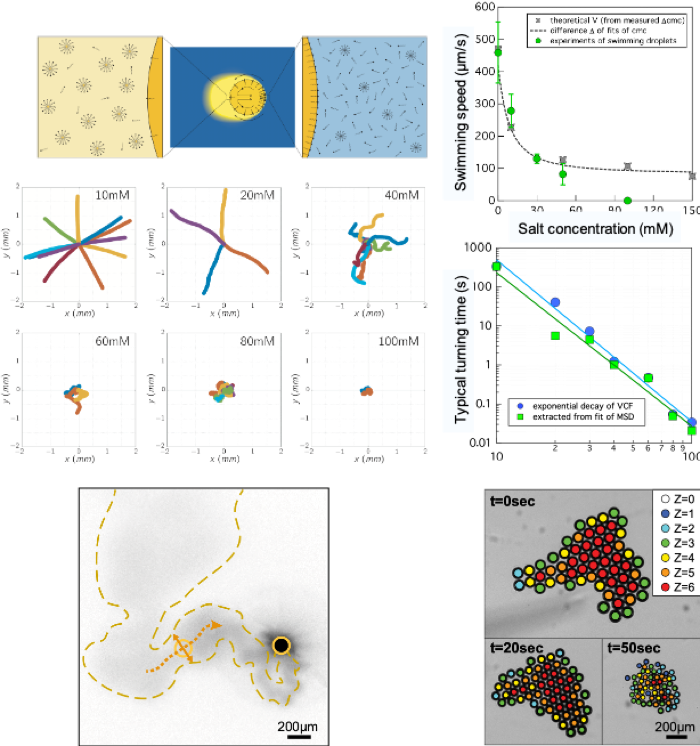
Here we explain the molecular engine of droplet motion that gives rise to their persistent random walk. This result allows us to tune their swimming speed and turning frequency over a range that is much broader than that of solid active particles. The tunability of their motion provides an excellent platform for studying non-equilibrium phenomena. For instance, we observe that droplets organize into dynamical clusters of a given size, much like bacterial colonies.