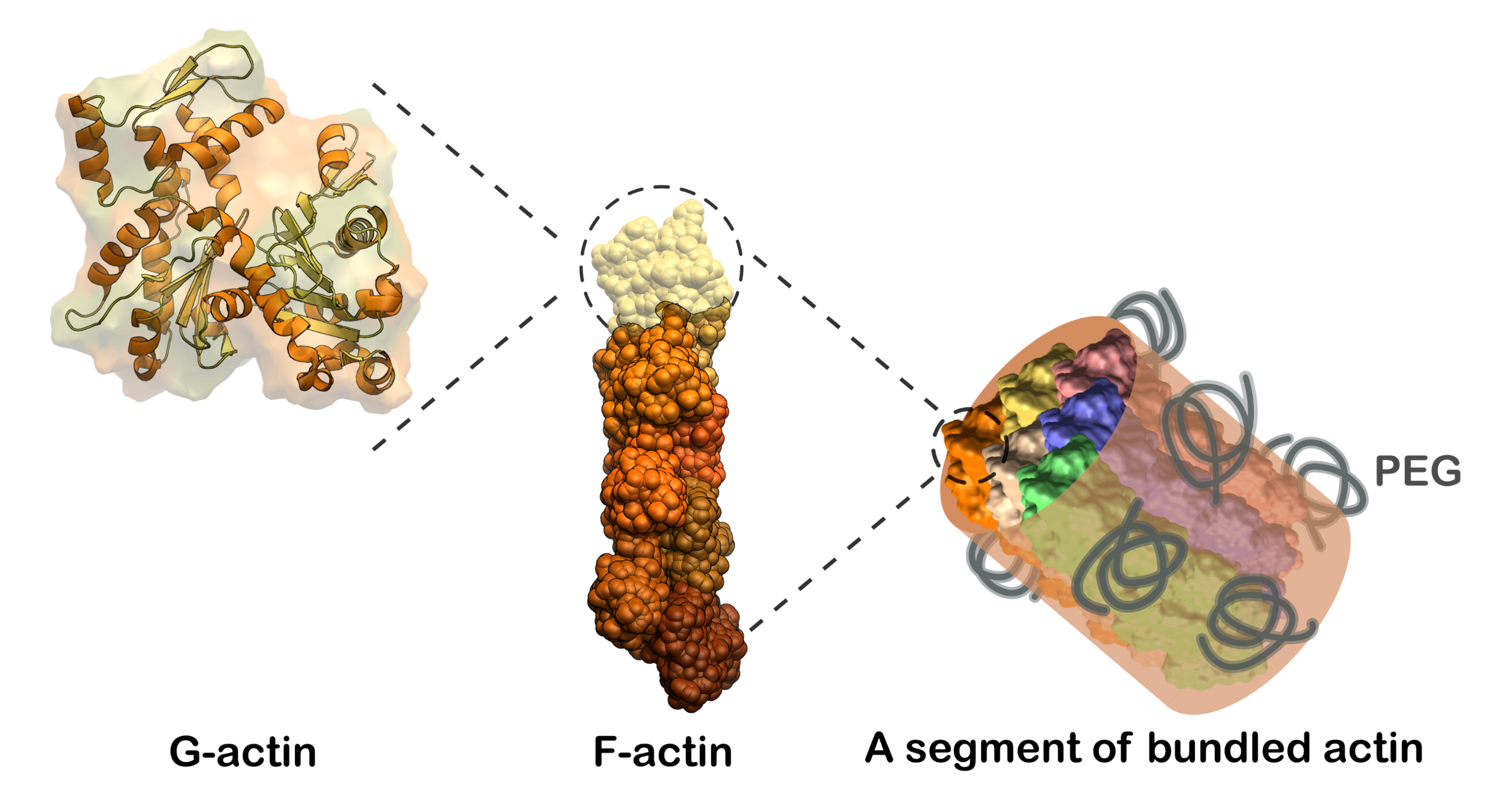 The cytoskeletal component actin plays a crucial role in various cellular functions, including cell shape regulation and intracellular transport, by forming filaments and networks. Despite the current understanding of actin's morphological versatility, the impact of crowded environments—specifically how actin filaments organize into bundles and how this organization changes the protein secondary structure—remains under-explored. Here, we used two-dimensional infrared (2D IR) spectroscopy and structure based spectral calculations to map out structural changes of actin filaments under two degrees of crowding and bundling.
The cytoskeletal component actin plays a crucial role in various cellular functions, including cell shape regulation and intracellular transport, by forming filaments and networks. Despite the current understanding of actin's morphological versatility, the impact of crowded environments—specifically how actin filaments organize into bundles and how this organization changes the protein secondary structure—remains under-explored. Here, we used two-dimensional infrared (2D IR) spectroscopy and structure based spectral calculations to map out structural changes of actin filaments under two degrees of crowding and bundling.
UT Austin MRSEC
DMR-1720595