 Wisconsin MRSEC researchers have developed a method to synthesize materials with precisely controlled crystal structures, even when the same atoms could arrange themselves into a different structure with nearly the same energy. The methods allow them to make highly perfect films of cubic aluminum oxide (Al2O3) with widespread applications in electronic materials, catalysis, and surface passivation. The cubic crystal structure is hard to make because it is similar in energy to a hexagonal crystal structure of the same atoms that lacks the desirable properties.
Wisconsin MRSEC researchers have developed a method to synthesize materials with precisely controlled crystal structures, even when the same atoms could arrange themselves into a different structure with nearly the same energy. The methods allow them to make highly perfect films of cubic aluminum oxide (Al2O3) with widespread applications in electronic materials, catalysis, and surface passivation. The cubic crystal structure is hard to make because it is similar in energy to a hexagonal crystal structure of the same atoms that lacks the desirable properties.
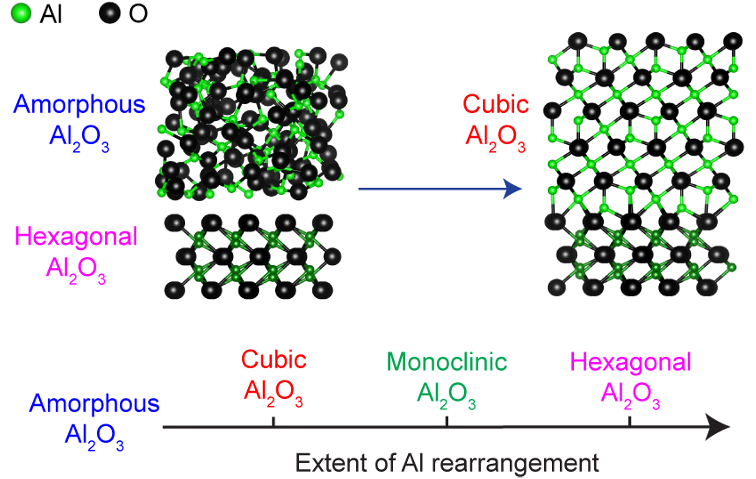
The approach used solid-phase epitaxy to crystallize Al2O3 from a disordered, amorphous precursor at low temperatures, which avoids forming the undesirable crystal structure. Computer simulations show that the desirable cubic crystal forms because the atoms in the disordered precursor move less to get to the cubic structure than to the hexagonal structure. The combination of experiments and simulations suggest that solid-phase epitaxy may be a general path to synthesizing selected crystal structures, even when there are several competing crystal structures.