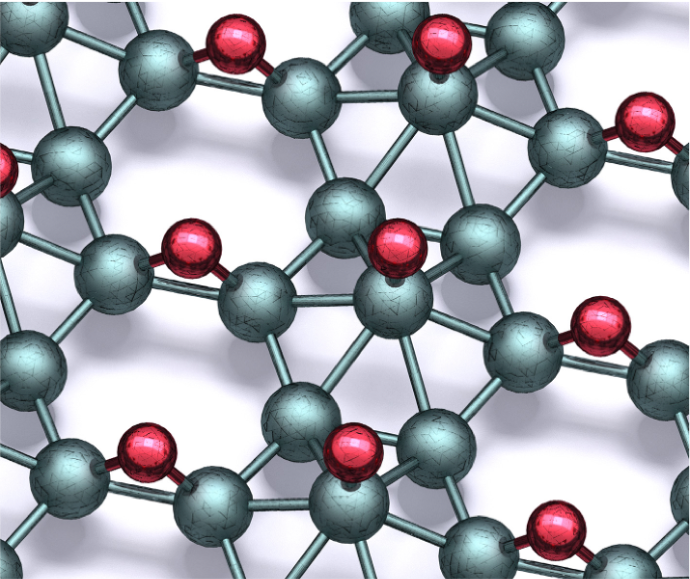
 Polymorphs of two-dimensional boron, or borophene, have attracted significant attention because of their diverse superlattice structures and correlated electron properties. However, ordered chemical modification of borophene has not previously been reported. In a three PI collaboration within NU-MRSEC IRG-1, “borophane” polymorphs have been synthesized by hydrogenating borophene with atomic hydrogen in ultrahigh vacuum. Borophane polymorphs are metallic and can be reversibly returned to pristine borophene through thermal desorption of hydrogen. Hydrogenation also provides chemical passivation as borophane is shown to be resistant to oxidation for multiple days following ambient exposure, thus enabling full exploration of its potential for nanoelectronics, energy storage, sensors, and quantum information technology.
Polymorphs of two-dimensional boron, or borophene, have attracted significant attention because of their diverse superlattice structures and correlated electron properties. However, ordered chemical modification of borophene has not previously been reported. In a three PI collaboration within NU-MRSEC IRG-1, “borophane” polymorphs have been synthesized by hydrogenating borophene with atomic hydrogen in ultrahigh vacuum. Borophane polymorphs are metallic and can be reversibly returned to pristine borophene through thermal desorption of hydrogen. Hydrogenation also provides chemical passivation as borophane is shown to be resistant to oxidation for multiple days following ambient exposure, thus enabling full exploration of its potential for nanoelectronics, energy storage, sensors, and quantum information technology.