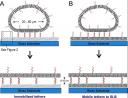
 We have developed two strategies forÂ’ preparing tethered lipid bilayer membrane patches on solid surfaces by DNA hybridization.Â’ In the first strategy, single-stranded DNA strands are immobilized by click chemistry to a silica surface, whose remaining surface is passivated to prevent direct assembly of a solid supported bilayer.Â’ Then giant unilamellar vesicles (GUVs) displaying the anti-sense strand based on a DNA-lipid conjugate are allowed to tether, spread, and rupture to form tethered bilayer patches.Â’ In the second strategy, a supported lipid bilayer displaying the DNA-lipid conjugate is first assembled on the surface. Then GUVs displaying the anti-sense strand are allowed to tether, spread andÂ’ rupture to form tethered bilayer patches.Â’ The essential difference between these methods is that the tethering hybrid DNA is immobile in the first, while it is mobile in the second. In the case of mobile tethers, different length DNA hybrids lead to lateral segregation by length that can be visualized by fluorescence interference contrast microscopy.Â’ Both architectures offer flexible platforms for the assembly of lipid bilayers at well-defined distances from the solid support.
We have developed two strategies forÂ’ preparing tethered lipid bilayer membrane patches on solid surfaces by DNA hybridization.Â’ In the first strategy, single-stranded DNA strands are immobilized by click chemistry to a silica surface, whose remaining surface is passivated to prevent direct assembly of a solid supported bilayer.Â’ Then giant unilamellar vesicles (GUVs) displaying the anti-sense strand based on a DNA-lipid conjugate are allowed to tether, spread, and rupture to form tethered bilayer patches.Â’ In the second strategy, a supported lipid bilayer displaying the DNA-lipid conjugate is first assembled on the surface. Then GUVs displaying the anti-sense strand are allowed to tether, spread andÂ’ rupture to form tethered bilayer patches.Â’ The essential difference between these methods is that the tethering hybrid DNA is immobile in the first, while it is mobile in the second. In the case of mobile tethers, different length DNA hybrids lead to lateral segregation by length that can be visualized by fluorescence interference contrast microscopy.Â’ Both architectures offer flexible platforms for the assembly of lipid bilayers at well-defined distances from the solid support.