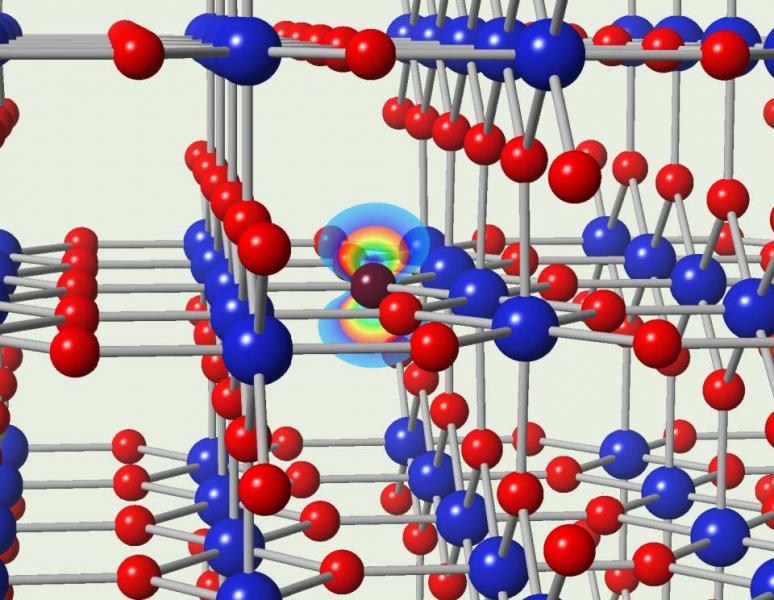
Titanium dioxide (TiO2) has been intensively pursued as a photocatalyst for applications ranging from the degradation of pollutants to water splitting. Currently, the rutile and anatase phases absorb light only in the ultraviolet, which limits their effectiveness. To address this major challenge, nitrogen doping is known to lead to visible-light absorption, but the mechanisms behind the process have remained unexplained. Recent computational work in IRG-2 have revealed that impurity-band transitions in the yellow-green range of the spectrum can be unambiguously attributed to substitutional nitrogen, and not to other forms of nitrogen. This result opens up the design visible-light photocatalysts based on TiO2.