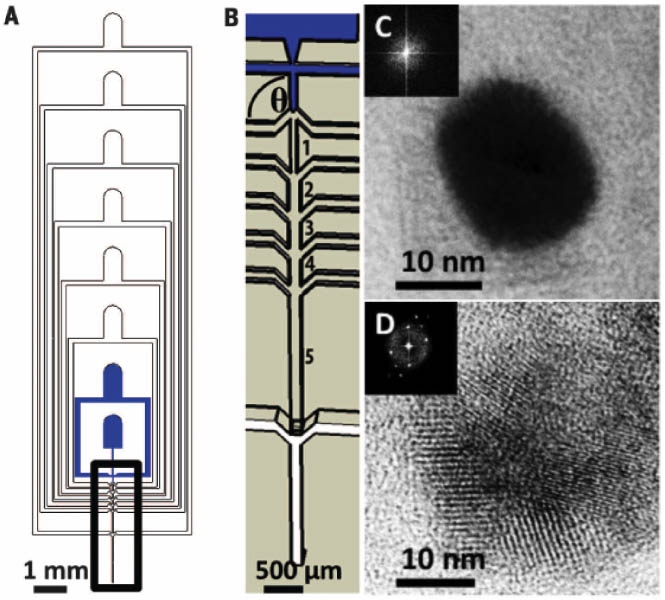
Table salt (NaCl) is always crystalline!But wait!!
A Harvard MRSEC team reported, in the August 29, 2015 issue of Science, a microfluidic spray drier, the
nebulator (a,b), that forms very small drops of fluid that are spray dried. David Weitz, Frans Spaepen and Michael Brenner used the
microfluidic spray drier to make nanoparticles of virtually all materials,
including drugs and inorganic materials. Because the drops are so small, the
particles dry very rapidly and are kinetically trapped in an amorphous state,
providing a simple means to produce amorphous nanoparticles. The amorphous
particles are much more soluble, making them valuable for drug delivery. They
are also surprisingly stable because crystallization is kinetically delayed due
to their small size. Even something as ubiquitously crystalline as table salt
can be made amorphous; there are no lattice planes seen by TEM (c) until the electron beam heats the
nanoparticle to crystallize it (d).