Human embryonic stem cells (hESCs) hold vast promise in science and medicine because of their potential to replicate indefinitely and their capability to differentiate to any cell type found in the adult. Many environmental cues, including soluble factors and intercellular signals, affect hESC differentiation and self-renewal decisions. By integrating a variety of carefully synthesized materials, engineers at the University of Wisconsin have developed a culture system that precisely regulates the size and shape of hESC colonies by confining them to three-dimensional microwells, while providing desired soluble and immobilized chemical factors. By measuring growth and differentiation rates of hESC colonies of different sizes, the UW-MRSEC has identified an optimum self-renewing colony size of 100-200 um diameter; smaller colonies grow slowly, while larger colonies exhibit undesired spontaneous differentiation. Results of this study illustrate the importance of regulating intercellular interactions by designing materials-based strategies to control hESC growth and differentiation. In addition, the microwell culture system reduces variability in hESC cultures arising from colony size and shape, and provides a much-needed consistent supply of undifferentiated cells for emerging science and engineering applications.
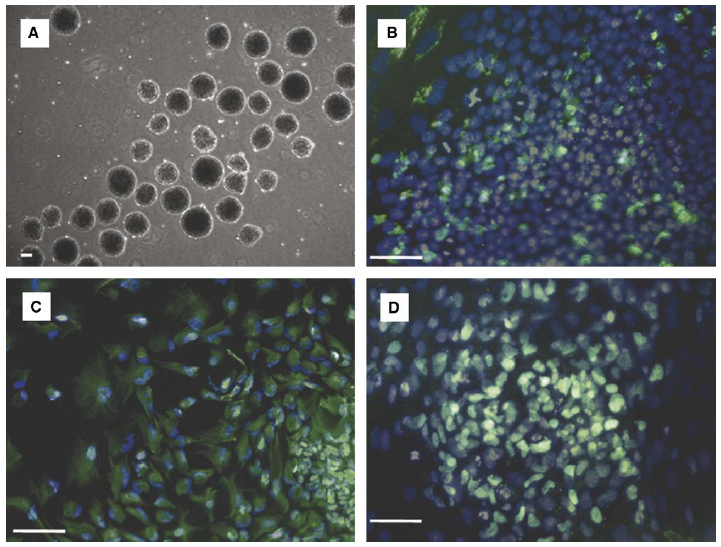
Embryoid bodies from hESCs cultured for 11 days in 50 mm deep, 100 mm/side microwells retain pluripotency. EBs were cultured in suspension 8 days in UMF to allow differentiation before plating on gelatin and culturing 8 days in UMF medium with 5% FBS. (A) Day 0 embryoid bodies on gelatin surface prior to attachment and differentiation. Pluripotency was determined through identification cells in each of the three embryonic germ layers via Hoechst nuclear dye staining (blue) and immunocytochemistry (green) targeting: (B) a-fetoprotein (endoderm); (C) nestin (ectoderm); and (D) brachyury (mesoderm). Scale bars: 100 mm.