 We recently described the first example of a photoactive polymersome, which was formed by incorporating a protein in the aqueous interior and a meso-to-meso ethyne-bridged bis[(porphinato)zinc] (PZn2) chromophore in the membrane.1 We initially investigated the encapsulation of horse spleen ferritin (HSF) or iron-free apoferritin (HSAF) inside a polyethylene oxide-polybutadiene (PEO30-PBD46, denoted OB29) diblock copolymer vesicle. Ferritins are large iron-storage proteins (MW ≈ 440 kDa) with twenty-four 4-helix bundle subunits that form a 12-nm sphere with an 8-nm inner cavity. Ferritin sequesters up to 4500 iron atoms as a hydrous ferric oxide mineral core and can serve as a magnetic resonance imaging contrast agent. We observed that ferritin incorporation drives the preferred polymersome morphology away from simple spheres towards asymmetric morphologies. Although some polymersomes remain spherical, increases in ferritin concentration lead to an increased frequency of polymersomes in non-spherical structures. The simultaneous incorporation of PZn2 into the vesicle hydrophobic membrane imparts sensitivity to focused light of near-UV to near-IR wavelengths. Confocal laser scanning microscopy (CLSM) imaging of polymersomes loaded with both ferritin and PZn2 at excitation wavelengths (488, 543, or 633 nm) where PZn2 absorbs strongly caused many of the vesicles to undergo irreversible morphological changes ranging from formation of new bends or ‘arms’ and budding of smaller vesicles to total polymersome destruction (Figure 1). Similar results were seen during imaging by widefield fluorescence microscopy using a mercury arc lamp. Observations of budding from CLSM suggest that the shape changes are caused by localized heating due to sample absorption of focused light within the membrane.
We recently described the first example of a photoactive polymersome, which was formed by incorporating a protein in the aqueous interior and a meso-to-meso ethyne-bridged bis[(porphinato)zinc] (PZn2) chromophore in the membrane.1 We initially investigated the encapsulation of horse spleen ferritin (HSF) or iron-free apoferritin (HSAF) inside a polyethylene oxide-polybutadiene (PEO30-PBD46, denoted OB29) diblock copolymer vesicle. Ferritins are large iron-storage proteins (MW ≈ 440 kDa) with twenty-four 4-helix bundle subunits that form a 12-nm sphere with an 8-nm inner cavity. Ferritin sequesters up to 4500 iron atoms as a hydrous ferric oxide mineral core and can serve as a magnetic resonance imaging contrast agent. We observed that ferritin incorporation drives the preferred polymersome morphology away from simple spheres towards asymmetric morphologies. Although some polymersomes remain spherical, increases in ferritin concentration lead to an increased frequency of polymersomes in non-spherical structures. The simultaneous incorporation of PZn2 into the vesicle hydrophobic membrane imparts sensitivity to focused light of near-UV to near-IR wavelengths. Confocal laser scanning microscopy (CLSM) imaging of polymersomes loaded with both ferritin and PZn2 at excitation wavelengths (488, 543, or 633 nm) where PZn2 absorbs strongly caused many of the vesicles to undergo irreversible morphological changes ranging from formation of new bends or ‘arms’ and budding of smaller vesicles to total polymersome destruction (Figure 1). Similar results were seen during imaging by widefield fluorescence microscopy using a mercury arc lamp. Observations of budding from CLSM suggest that the shape changes are caused by localized heating due to sample absorption of focused light within the membrane.
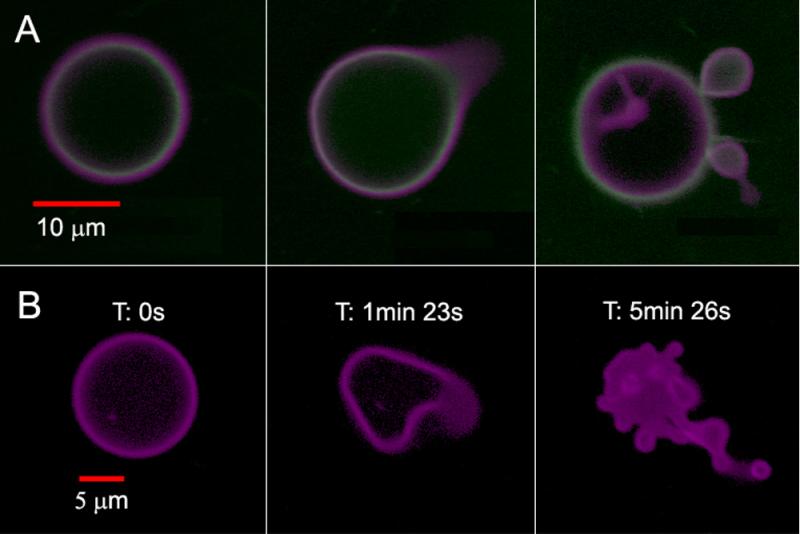
figure: Confocal micrographs of polymersomes that membrane-disperse PZn2 (purple) and encapsulate HSAF obtained in continuous scanning mode. (A) BODIPY-FL-labeled HSAF (green, 3 mg/mL) + PZn2 vesicle, imaged using two lasers simultaneously (488 nm, 543 nm). Images proceed in time, left to right, over a period of ~5 min. (B) Unlabeled HSAF (1.5 mg/mL) + PZn2 vesicle. Vesicle imaged using three lasers simultaneously (488, 543, 633 nm). Final image of the degraded structure was not in the same plane as the original vesicle.