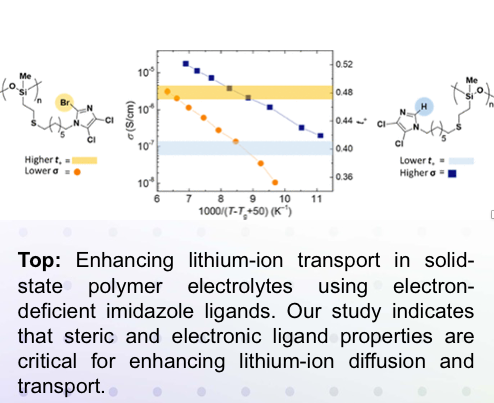
Intellectual merit: Solid-state polymer electrolytes offer a safer alternative to traditional lithium-ion batteries based on organic electrolytes. However, current benchmark polymer electrolytes lack ion transport selectivity (t+ = 0.2) which limits their commercial use. We demonstrate the enhancement of lithium-ion transport (t+ = 0.48) of PMS-based polymers by taking advantage of the steric and electronic properties of imidazole ligands. This study highlights the importance of weak cation-polymer binding to obtain high Li+ transport numbers.
Broader Impacts: Understanding how ligand choice influences total ionic conductivity and lithium-ion transport will enable the development of next-generation solid-state polymer electrolytes with superior performance. Such materials can potentially be used in energy storage applications on a commercial scale.