 Liquid crystals are soft materials which see frequent use in optical displays and other smart devices. This is because they can change their optical properties (such as light transmission and polarization) when an electric field is applied. This allows them to selectively block or transmit light, creating the pixels that form images on the screen. Similarly, nanoparticles are materials that can have different optical properties that depend on their size.
Liquid crystals are soft materials which see frequent use in optical displays and other smart devices. This is because they can change their optical properties (such as light transmission and polarization) when an electric field is applied. This allows them to selectively block or transmit light, creating the pixels that form images on the screen. Similarly, nanoparticles are materials that can have different optical properties that depend on their size.
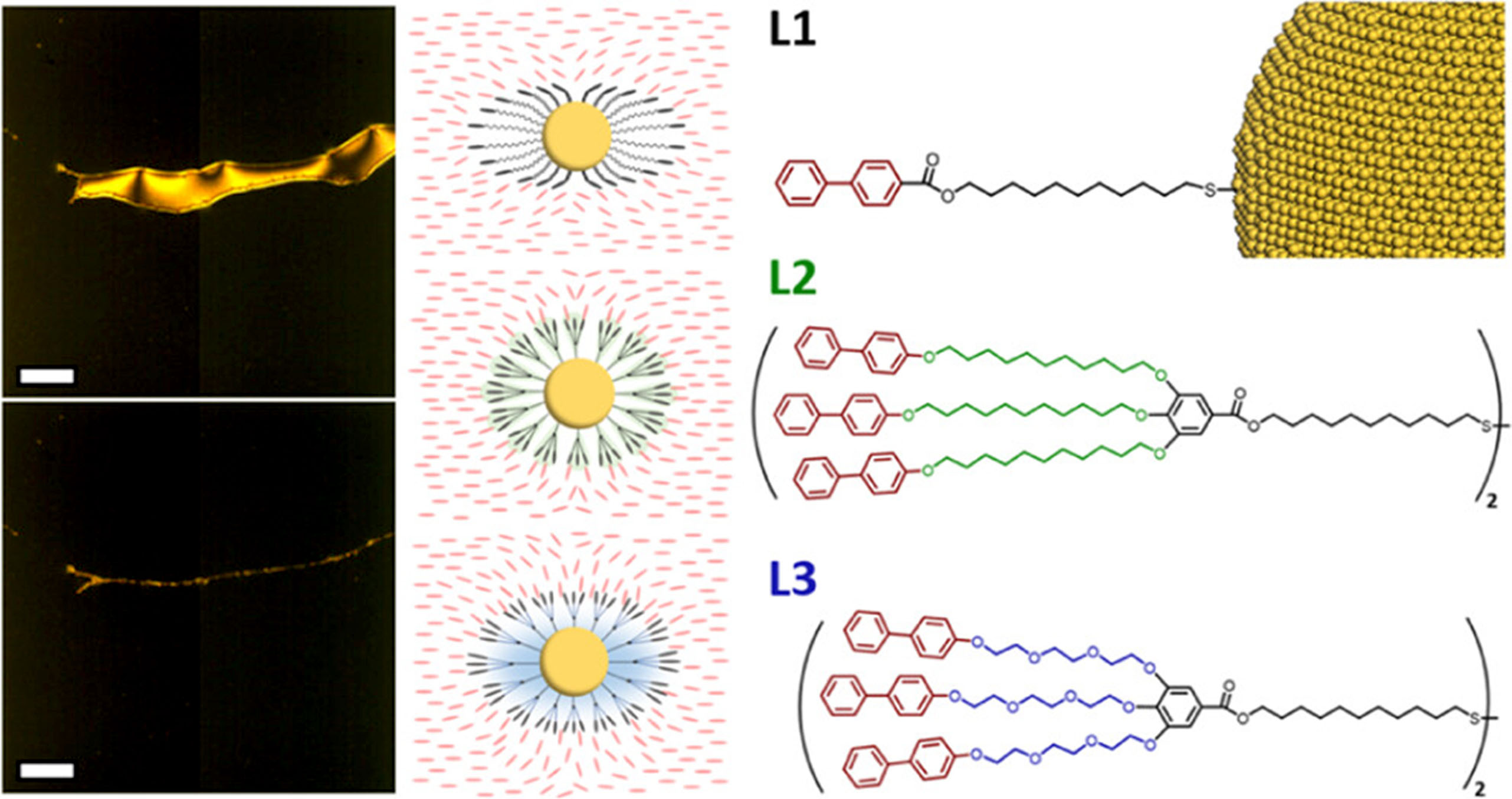
In this work, Penn researchers have developed new liquid crystal-nanoparticle hybrid systems. They have integrated specially synthesized molecules known as “dendritic promesogenic ligands” that can attach to the nanoparticles.
These dendritic ligands assist in the dynamic assembly of nanoparticles (NPs) driven by the alignment and equilibration of mesogens. These ligands significantly enhance the shelf-life and thermal stability of nanoparticle assemblies compared to linear ligands. Gold NPs capped with OEG-chained dendrimers remain stable in 5CB liquid crystal for 6 months at room temperature and over 10 hours at 50°C. The synthesis of dendritic ligands is highly modular and can be applied to NPs with different dimensions and properties.
This modular approach can be applied to various NPs, enabling the development of tunable optical displays and responsive materials.
Image Caption: (left) Polarized optical microscopy images showing that the ligands can align nanocrystal assembly, (middle) illustration of the proposed ligand arrangement, (right) Dendritic Promesogenic Ligands used in the study
Ning, Y. F., Liu, Z., Yang, S. S., Morimitsu, Y., Osuji, C. O. and Murray, C. B., Design of Dendritic Promesogenic Ligands for Liquid Crystal- Nanoparticle Hybrid Systems. Chemistry of Materials 35 (9), 3532-3544 (2023) 10.1021/acs.chemmater.3c00057/ http://dx.doi.org/10.1021/acs.chemmater.3c00057