Colloidal gels are formed by aggregation of particles into a percolating network in liquid media. Although colloidal gels exhibit self-supporting, solid-like properties that underlie the design of a wide range of materials, how this class of soft solids forms is not yet fully understood. In particular, the relative importance of thermodynamic and dynamic 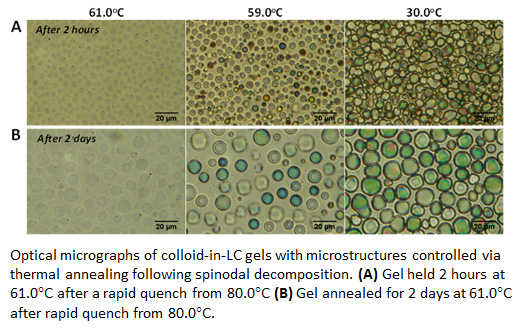 (non-equilibrium) phenomena underlying the formation of gels is debated. Over the past year, IRG3 of the Wisconsin MRSEC has discovered a new pathway leading to formation of colloidal gels in thermotropic liquid crystals (LCs). In contrast to gels formed from isotropic solvents, the mechanical properties of the gels formed in LCs reflect the long range orientational ordering and the associated elasticity of the LC phase. These attributes and others make this class of materials rich in fundamental phenomena and also technologically promising. In particular, whereas two previous methods of synthesis leading to colloid-in-LC gels have been reported, both involve non-equilibrium pathways. In contrast, in the pathway discovered by IRG3, the onset of formation of the colloidal microstructure is driven by a thermodynamic instability (spinodal), and gelation occurs upon nucleation of nematic LC within the colloidal microstructure formed via spinodal decomposition. Identification of a thermodynamic pathway leading to formation of the CLC gel is significant because it enables rationale design of the microstructure and responsive properties of this technologically promising class of soft solids (see Figure). Ongoing studies are investigating these materials for use in biological sensors and as tunable substrates for cell culture.
(non-equilibrium) phenomena underlying the formation of gels is debated. Over the past year, IRG3 of the Wisconsin MRSEC has discovered a new pathway leading to formation of colloidal gels in thermotropic liquid crystals (LCs). In contrast to gels formed from isotropic solvents, the mechanical properties of the gels formed in LCs reflect the long range orientational ordering and the associated elasticity of the LC phase. These attributes and others make this class of materials rich in fundamental phenomena and also technologically promising. In particular, whereas two previous methods of synthesis leading to colloid-in-LC gels have been reported, both involve non-equilibrium pathways. In contrast, in the pathway discovered by IRG3, the onset of formation of the colloidal microstructure is driven by a thermodynamic instability (spinodal), and gelation occurs upon nucleation of nematic LC within the colloidal microstructure formed via spinodal decomposition. Identification of a thermodynamic pathway leading to formation of the CLC gel is significant because it enables rationale design of the microstructure and responsive properties of this technologically promising class of soft solids (see Figure). Ongoing studies are investigating these materials for use in biological sensors and as tunable substrates for cell culture.