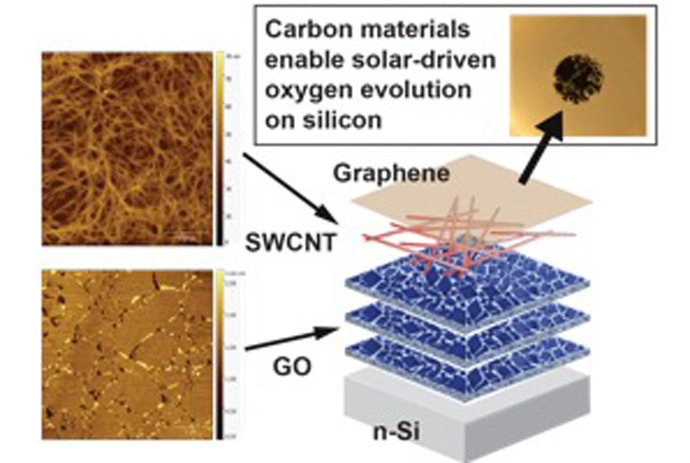
Solar water splitting converts solar energy into chemical fuels that can be easily stored and transported. Silicon is already used on a large scale for photovoltaics, but it is unstable in the electrolytes used for water oxidation. Here, graphene and carbon nanotubes, which are respectively single-atom-thick sheets and tubes of carbon, protect silicon anodes and enable water oxidation with increased efficiency. Holes, one type of excited charge carrier, are extracted from the silicon into the sorted semiconducting carbon nanotubes before loss can occur via recombination or corrosion. Unlike prior protective layers, these films contain no precious metals and can be deposited from solution under ambient conditions, providing a route towards large-scale, low-cost solar fuel production.
Nano Lett., 16, 7370 (2016)

Northwestern Materials Research Science and Engineering Center
NU-MRSEC advances world-class materials research, education, and outreach via active interdisciplinary collaborations within the Center and with external partners in academia, industry, national laboratories, and museums, both domestically and abroad.