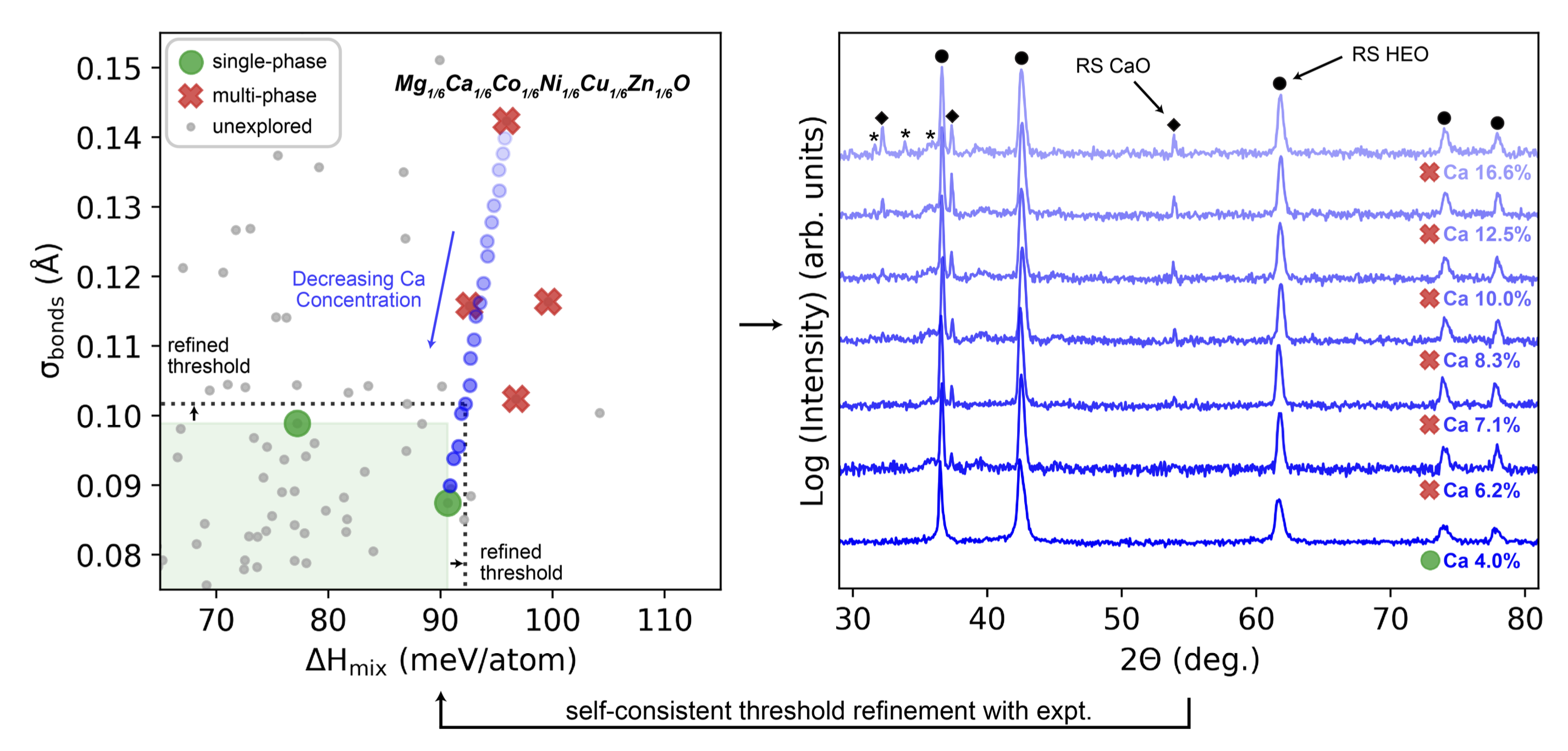
High-entropy materials shift the traditional materials discovery paradigm to one that leverages disorder, enabling access to unique chemistries unreachable through enthalpy alone. A MRSEC team has developed a high-throughput framework for discovering and understanding the single-phase formation of high-entropy oxides (HEOs) by integrating computation and experiment in a self-consistent feedback loop. To more rapidly explore rock salt composition space, the team utilizes CHGNet machine-learning interatomic potentials with impressive accuracy even in disordered systems.
Two computational descriptors resolve the single-phase stability for all eight equimolar rock salt HEO compositions explored to date and lead to the discovery of a novel non-equimolar rock salt HEO containing Ca. This collaborative workflow workflow is currently being applied to explore more complex crystal structures that may possess emerging property opportunities.

Penn State Center for Nanoscale Science (2020)
The Center for Nanoscale Science supports collaborative, interdisciplinary research efforts on nanoscale materials. Principal research activities are organized into two interdisciplinary research groups: 2D Polar Metals & Heterostructures and Crystalline Oxides with High Entropy. Center-initiated programs encourage collaborative partnerships with science museums and non-R1 universities as well as engagement in outreach, education, and workforce development initiatives.