 Nanocrystal gelation is a strategy to translate exceptional properties inherent to nanoscale building blocks into multiscale architectures and devices. However, available gelation methods are not easily adaptable across broad classes of nanocrystal systems since assembly is strongly reliant on specific surface chemistries.
Nanocrystal gelation is a strategy to translate exceptional properties inherent to nanoscale building blocks into multiscale architectures and devices. However, available gelation methods are not easily adaptable across broad classes of nanocrystal systems since assembly is strongly reliant on specific surface chemistries.
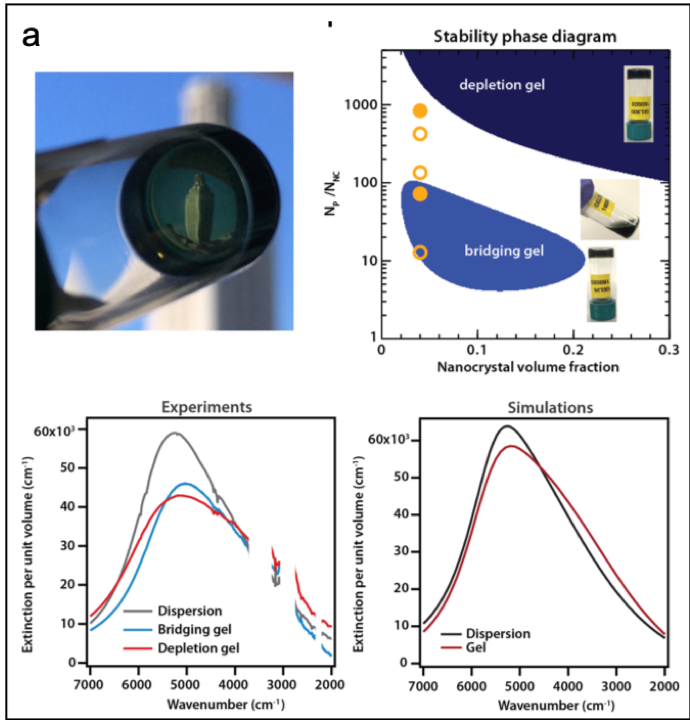
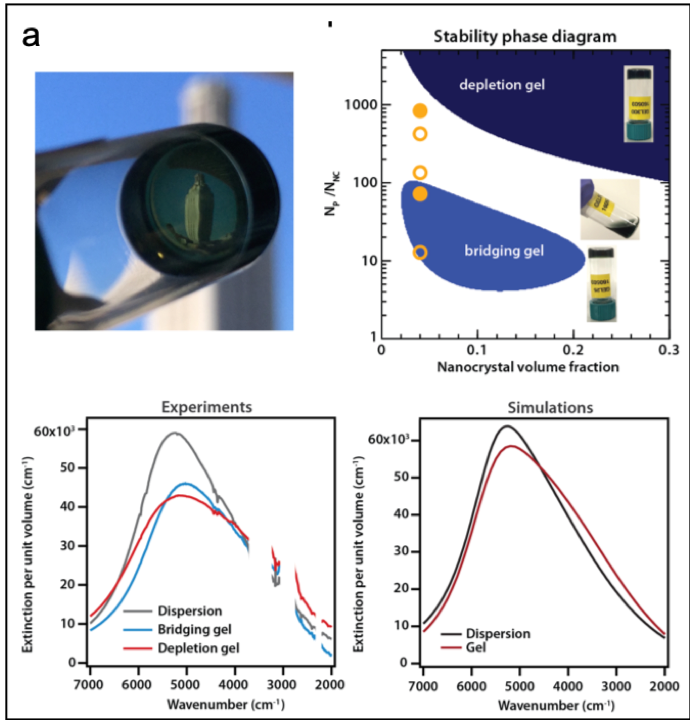
We designed an alternate gelation approach based on physical interactions instead of specific chemical linkages: depletion attractions balanced by electrostatic repulsions. We demonstrate its feasibility by assembling charge-stabilized tin-doped indium oxide nanocrystals in the presence of polyethylene glycol. Increasing the polymer-to-nanocrystal ratio and thereby the strength of attraction results in a phase behavior featuring two favorable gelation windows that are preceded by a fluid regime. Bridging effects dominate at low polymer concentration while depletion attractions drive gelation at higher concentration according to predictions from our unified theoretical model. Because our approach prevents nanocrystal fusion during assembly, we achieve gels that strongly absorb in the infrared with an optical response characteristic of both the discrete building blocks and the network architecture.