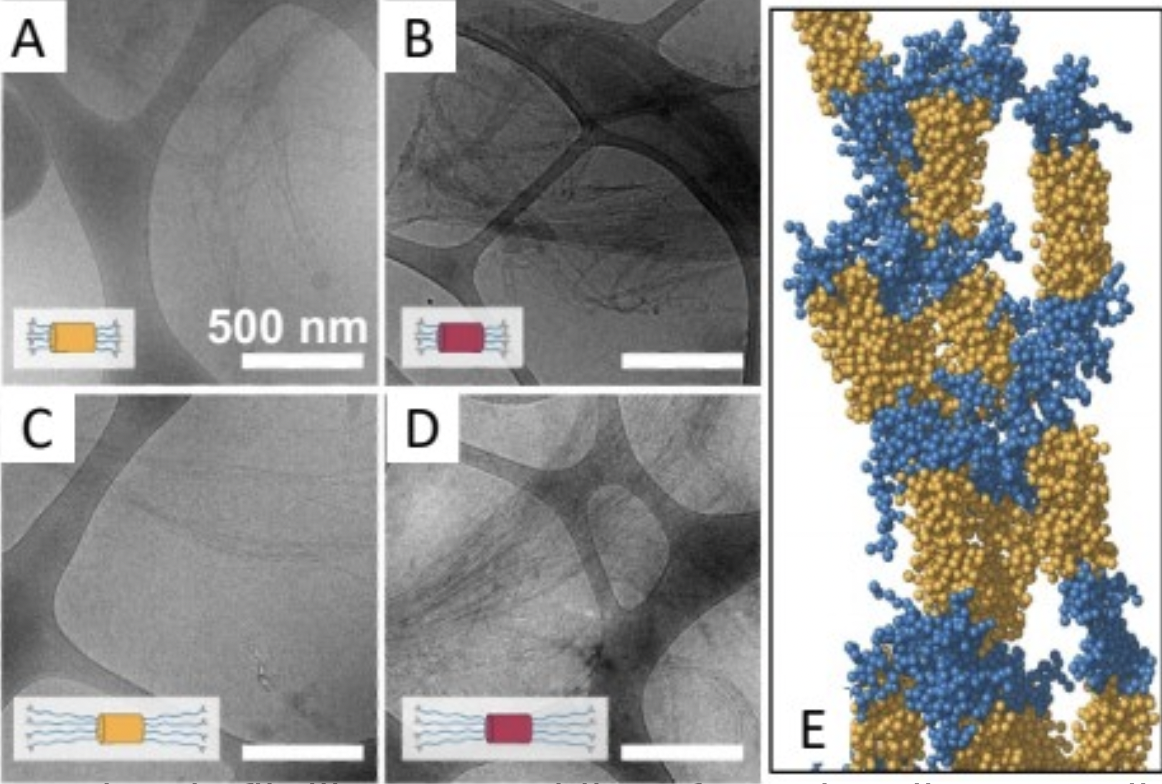
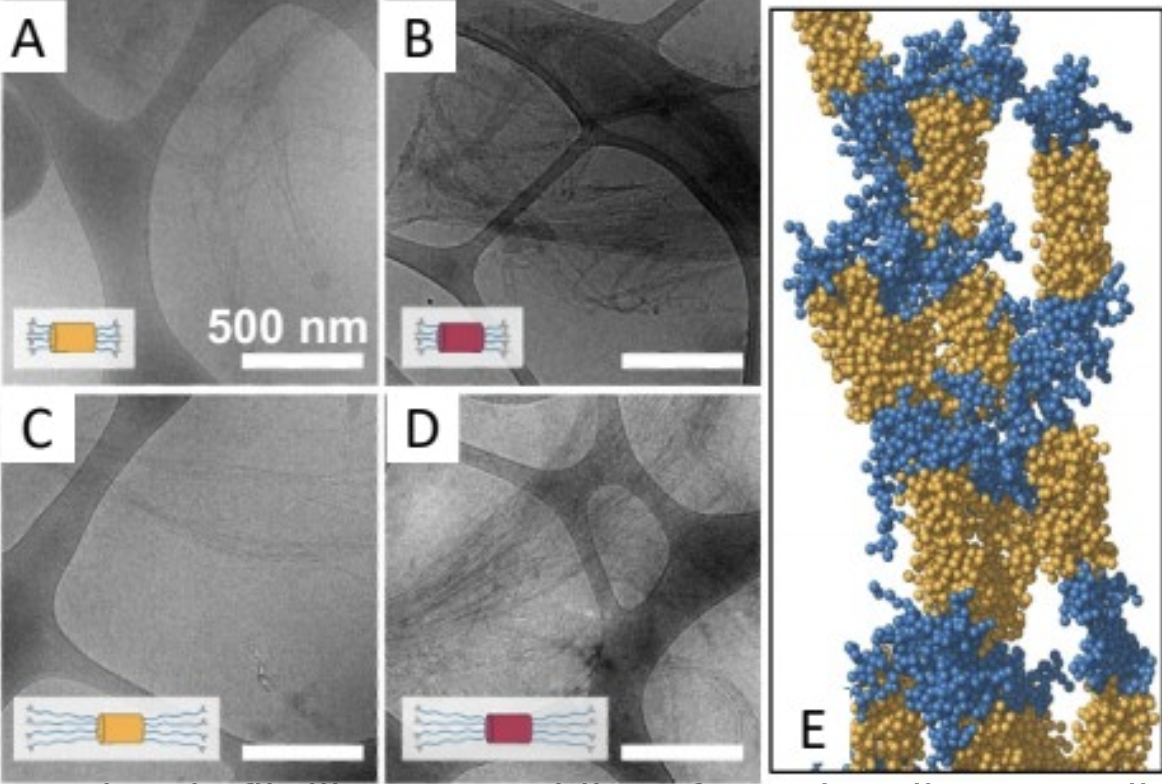
 The ability to thermally trigger a conformational changeand collapse in a constituent resilin-like protein (RLP) providesthe opportunity to build, and eventually move, a bundlemer nanostructure.
The ability to thermally trigger a conformational changeand collapse in a constituent resilin-like protein (RLP) providesthe opportunity to build, and eventually move, a bundlemer nanostructure.
Bundlemer peptides can still assemble into coiled coil bundles even with RLPs attached to all four constituent peptides in each bundle. This is a clear demonstration that biosynthesis can be used for bundlemer building block production for future study.
- This ability affords a mechanism for the formation (and disassembly) of bundlemer-based nanomaterials through temperature responsive proteins
- The ability to thermally trigger a conformational change/collapse in a constituent polymer/protein provides the opportunity to purposefully move a bundlemer nanostructure in future Aim 2 efforts.
- This is a major step towards success of Aim 2 in which nanostructures will be purposefully moved. The thermal collapse of the RLP can be used to both form structure (Aim 1) and to move structure (Aim 2).
- The successful biosynthesis from E. Coli of the bundlemer peptide and RLP constructs clearly affords future success in peptide/protein production with biosynthesis for scale up and study.

Center for Hybrid, Active, and Responsive Materials
UD CHARM advances foundational understanding of new materials driven by theoretical and computational predictions paired with cutting-edge experiments to enable the integration of unconventional, ultra-small, building blocks.