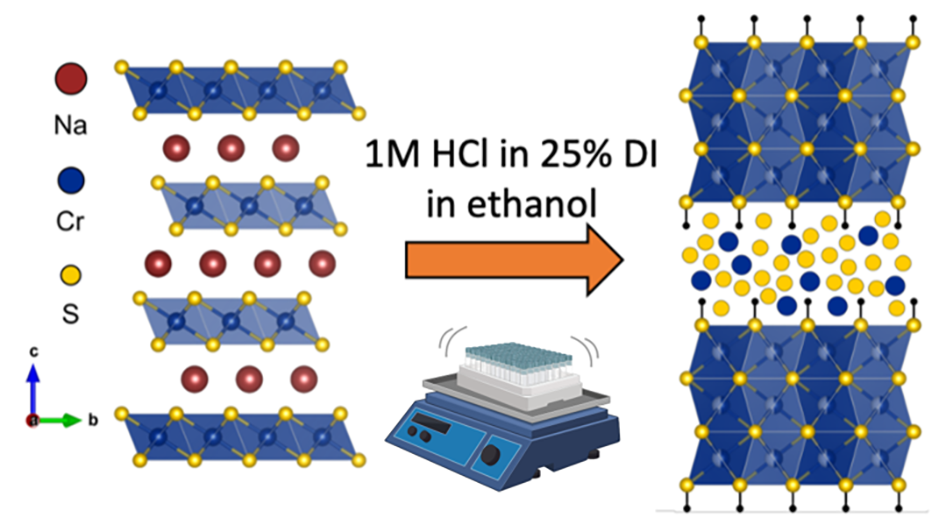
Princeton researchers have demonstrated that acid pre-treatment of NaCrS2 to form a new phase (named HxCrS2) results in significant improvements to the material’s performance as a sodium battery electrode. Acid exchange to remove sodium from NaCrS2 is accompanied by a phase transformation to a biphasic structure with alternating amorphous and crystalline layers. This unique structure allows for faster diffusion of Na+ and results in higher capacities that can be sustained over longer cycling lifetimes. Specifically, their new material features a capacity of 728 mAh/g that is maintained throughout cycling and is nearly double the reversible capacity of NaCrS2 in the same potential region. HxCrS2 also features a Na+ diffusion constant on the order of 10,000 times faster than in NaCrS2.

Princeton Center for Complex Materials
Established in 1994, the Princeton Center for Complex Materials is dedicated to exploring the frontiers of complexity in materials science. The Center supports two IRGs that will accelerate exploration of quantum technologies and biology-inspired materials.