Electrolyte gating may sound complex, but at its heart it’s a simple idea: use ions to do what transistors have done for decades but in a more powerful, energy-efficient way. For Chris Leighton, professor at the University of Minnesota’s Department of Chemical Engineering and Materials Science and Director of UMN’s MRSEC, this technique has opened the door to controlling materials in ways that once seemed impossible.
“These devices do what transistors have been doing for decades, but in a different, interesting way,” he says. “We use an electrical insulator, but we pick one that conducts ions, so when we put a voltage across it, ions move—we can shuttle them back and forth. By pushing them to an interface with a material of interest we can trigger electrochemical reactions, controlling the properties of materials at low voltages.”
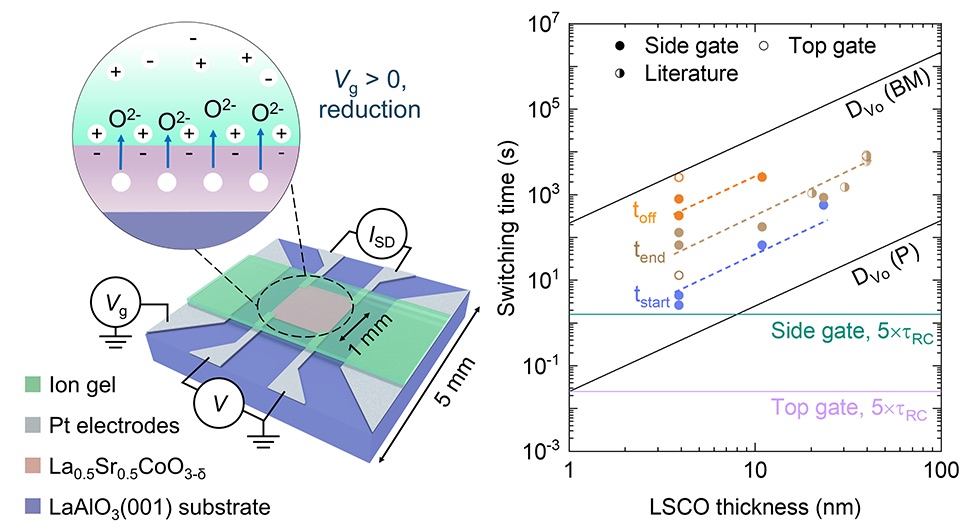
At the heart of this work is a perovskite oxide called La₀.₅Sr₀.₅CoO₃₋δ, or LSCO. It’s a cobalt-based material whose structure can easily gain or lose oxygen atoms, to the point that it can even change its whole atomic structure. This changes its electrical, magnetic, and optical properties dramatically, making LSCO an ideal platform for exploring how tiny voltages can control matter. And the team has shown this is possible with just one or two volts.
“This is really low power consumption,” says Leighton. “We can pull the oxygen out, which hugely changes the physical properties of the materials. Maybe the more surprising part is we can then switch the voltage to negative and push the oxygen back in; its reversible.”
Tackling the Two Big Challenges
Two issues have long stood in the way of practical devices based on these concepts: speed and endurance. “If you want to use this in a device—to change electronic or magnetic or optical properties—the two big problems with this are how fast can you make it respond, and how many times can you cycle it?”
On the endurance side, the team showed that by redesigning the devices and carefully controlling humidity, they could push past a barrier that had limited the field for years. “There’s a lot of papers out there that show only three or four cycles; literally one paper that shows 10, and here we made it to a 100,” Leighton says. “Most importantly, our work showed there’s a lot of stuff going on in these devices that we still don’t understand. If we do, I think it is very likely we can optimize this much further, to practical levels.”
To avoid confusion about whether the material or the electrical contacts were degrading, the researchers probed optical properties using infrared spectroscopy. However, the second challenge—speed—demanded a different approach. “Before we started doing this work, there were several papers published with response times in the 30 minute range,” Leighton says. His team and their collaborators carefully studied what actually limits the process and found that the bottleneck was oxygen motion in the LSCO itself, not the ions in the electrolyte.
By making ultra-thin films—just four nanometers thick—they were able to bring switching times down dramatically. “We were able to get the first signs of switching in 0.7 seconds,” Leighton said. “Some people might look at that and say, well, you’ve still got a long way to go … but we are at a factor of a hundred better than what has been achieved before. This is a pretty good step in a single piece of work.”
Path Toward Applications
Where could this lead? According to Leighton, the most immediate opportunities lie in optics and photonics. “Imagine you had a material and just by applying a voltage of one or two volts, you could change its refractive index on the fly. You could make an auto tuning lens, or you could make a beam steering device where light would come in at a certain angle, then go out at different angles controlled by the voltage.”
He and his collaborators see possibilities for tunable photonic devices, adaptable lenses, and novel telecommunications components. “If you can actively tune devices just by applying a voltage, then you can move resonance wavelengths around,” he said.
Next Steps and Collaborations
Moving forward, the team—which includes UMN MRSEC researchers Vivian Ferry, Kelsey Stoerzinger, Dan Frisbie, Rohan Chakraborty, and Jerry Liang—plans to chemically modify LSCO to enhance oxygen diffusion, borrowing strategies from solid oxide fuel cell devices. On endurance, Leighton said the focus is on better understanding the electrochemistry itself. “Everything depends very sensitively on the surface chemistry of the LSCO and the metal contacts.”
Collaboration has been key. “Over the years, in our MRSEC, we have collaborated with dozens of groups to take their materials, which are exciting and new, put them into this device platform to control them with voltages.” Examples include collaborations with Drexel, Penn State, and Stanford Universities.
What Drives the Work
Asked what excites him most about this work, Leighton didn’t hesitate. “The single most exciting thing for me is that we’re getting really close to applications. This has come from a lab idea, in its infancy, to something where we see this problem and that problem, but we’re knocking them down one-by-one, making big leaps. We’re not squeezing out factor of two improvements; these papers are more like factors of 100. I think that’s pretty exciting. You start to think it might be viable for real applications, that we really need.”
Read more about the team’s research:
Limits on Topotactic Transformation Speed in Electrolyte-Gate La0.5Sr0.5CoO3-δ Electrochemical Transistors
High Metal–Insulator Topotactic Cycling Endurance in Electrochemically Gated La0.5Sr0.5CoO3−δ Probed by Humidity-Dependent Operando Fourier Transform Infrared Spectroscopy